Pregnancy is no longer a surprise these days. Thanks to contraception, it is possible to have sex without conceiving a child. There are many different contraceptives, from regular contraceptive pills to one-off morning-after pills to non-hormonal contraceptives like condoms or the coil. Most of them are intended for use by women. In this text you can read all about the different types of contraception and how to use them.

Birth control
Consultation services
What is contraception?
Contraception is a method to prevent an unplanned pregnancy. There are several ways to do this. Some contraceptives (adhesion barriers) prevent sperm from reaching the uterus. Other contraceptives cause the conditions in the female genitalia to change, making a pregnancy impossible.
What forms of contraception are there?
We can roughly divide contraception into two main types: hormonal contraceptives and non-hormonal contraceptives. There is also a third option: definitive contraception (sterilisation).
Get treated without the inconvenience of waiting rooms. How does Dokteronline work?
You choose your treatment
Learn about the options and choose a treatment that suits your needs.
We'll guide you every step of the way
A doctor will review your medical questionnaire and send your prescription to an affiliated pharmacy.
Hassle-free home delivery
Your parcel will be delivered to your doorstep quickly and easily.
What types of hormonal contraceptives are there?
The contraceptive pill, also known simply as ‘the pill’, is one of the most commonly used contraceptives. The pill works on the basis of hormones. Most contraceptive pills are what is called a ‘combination pill’, containing both female sex hormones oestrogen and progestogen. These birth control pills are dosed in such a way that they inhibit ovulation, so that no eggs are released. In addition, the hormones change the structure of the uterine (mouth) mucosa. This makes it more difficult for sperm cells to penetrate into the uterus and any fertilised egg will not be able to implant itself.
What types of contraceptive pills are there?
There are different types of contraceptive pills to choose from, which can sometimes cause some confusion. The various types of pills are listed below:
- Single-phase pill: For this type of pill, all the tablets in the strip have the same composition. Each pill therefore contains the same dosage of the active ingredients. If the single-phase pill contains less than 30 mg of oestrogen, it is known as a sub-30-pill. If the pill contains 30 to 50 mg of oestrogen, it is known as a sub-50 pill. The less oestrogen a pill contains, the less chance there is of side effects.
- Multi-phase pill: With this type of pill, the dosage of the active ingredients differs from one pill to the next. One pill contains more oestrogen or progestogen than the other. The multi-stage pill mimics the natural hormonal balance of a menstrual cycle. The differences in dosage can be recognised by the different tablet colours.
- Mini pill: There are also contraceptive pills that contain only one hormone (progestogen). These so-called mini pills are lighter, less likely to cause side effects and can also be used if you are breastfeeding.
How do you use contraceptive pills?
Contraceptive pills are a very reliable contraceptives, provided you take them in the right way. Use of the pill differs with each type. With most contraceptive pills you take one tablet every day for three weeks. This is followed by a so-called stop week: Seven days in which you do not take a pill. During this week there is bleeding that looks like a menstrual period, but which is generally quite light. This is called a withdrawal bleed. You are also protected against pregnancy during the stop- week. There are also pills for which there is no stop-week. You take a pill every day until the strip is empty and then immediately proceed to the next strip. Depending on the type of pill, a withdrawal bleed can still occur.
The contraceptive pill is quickly worked out. If you stop using this contraceptive, you will be fertile again almost immediately.
Contraceptive pill for other purposes
Sometimes women take the pill for reasons other than birth control. For example, because they suffer from menstrual disorders, such as heavy bleeding, severe menstrual pain or a very irregular menstrual pattern. The pill replaces natural menstrual periods with a mild, regular withdrawal bleeding, which reduces these complaints.
Women who take the pill can shift their periods. This can be useful, for example, if you need surgery, go on holiday, have a sporting event or if your period is inconvenient for some other reason.
Emergency contraceptive pill or morning-after pill
The emergency contraceptive pill is not the same as a regular contraceptive pill. The so-called ‘morning-after pill’ is only meant to prevent a pregnancy after unprotected sexual intercourse. The emergency contraceptive pill contains a certain amount of hormones that inhibit ovulation and/or prevent a fertilised egg from settling in. This means that there can be no pregnancy. However, the tablet should be taken as soon as possible: preferably within twelve hours, but in any case within three to five days after unprotected intercourse.
Other types of hormonal contraceptives
- Contraceptive injection: Women who find it difficult to take a pill every day can opt for a contraceptive injection. The hormones are injected into the body, after which no pregnancy can occur for 12 weeks. The contraceptive injection is administered by a doctor or nurse.
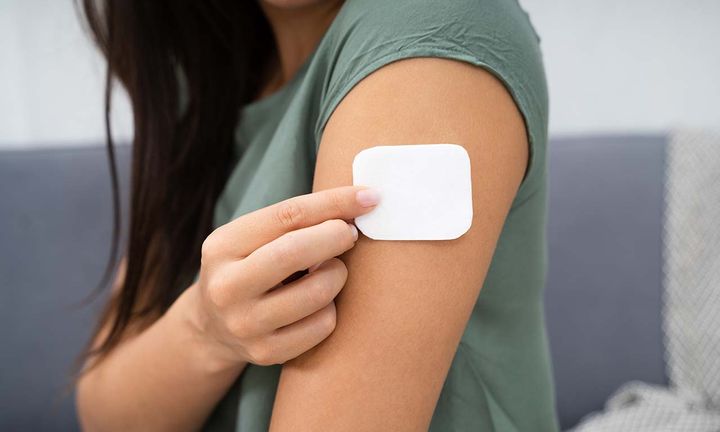
- Contraceptive implant: A hormone implant consists of a small implant that is placed under the skin in the upper arm. The implant releases a small dose of hormones every day. This means that you are continuously protected against pregnancy for a period of three years. The contraceptive implant is applied by a doctor or nurse.
- Contraceptive patches: When using a contraceptive patch, the hormones are absorbed into the blood through the skin. The patch is replaced once a week. After three weeks there will be a stop-week. During this week, a withdrawal bleed is triggered.
- Vaginal ring: A vaginal ring releases hormones into the mucous membrane of the vagina. The ring remains in place for three weeks, after which a stop-week follows. The ring is easy to insert and remove. The action can be compared to inserting a tampon.
- Hormone coil: A ‘coil’ is also known as an intrauterine device or IUD. It consists of a small, plastic anchor that is placed in the uterus by a doctor. The coil releases a small amount of progestogen every day. This means that you are continuously protected against pregnancy. The hormone coil should be replaced after 5 years.
What types of non-hormonal contraceptives are there?
There are also ways to prevent pregnancy without using hormones. Adhesion barriers such as condoms and pessaries are very suitable for this purpose. Another non-hormonal contraceptive is the copper coil. Non-hormonal contraceptives do not cause side effects and are safe for use by women who are breastfeeding.
- Adhesion barriers: An adhesion barrier prevents sperm from reaching the uterus. These contraceptives literally form a barrier between the sperm and the uterus. There are different types:
- Condom: The condom is the only non-definitive contraceptive for a man for the time being. Condoms are safe as long as they are used according to instructions. The advantage of a condom is that this contraceptive also protects against an STD (sexually transmitted disease). Condoms come in many shapes and sizes. Choose a variant that fits well and feels good.
- Female condom: The female condom consists of a kind of bag with a rubber ring. Insert the condom (up to 24 hours) into the vagina before sexual intercourse. This is useful if you do not want to interrupt sexual activities, as with using a male condom. The female condom also protects against STDs.
- Pessary: A pessary is a rubber cap that is placed over the cervix. This can be fitted from two hours before sexual intercourse. Always use the pessary in combination with a spermicide paste. After sexual intercourse, the pessary must be kept in the vagina for another 6 hours. Only then is it certain that all sperm cells have been killed and the cap can be safely removed.
- Copper coil: A copper coil is a small, anchor-shaped object that is placed in the uterus. The coil continuously emits a small amount of copper. This makes sperm inactive, so no fertilisation can take place. In this way, a copper coil protects against an unwanted pregnancy for about five to ten years. The spiral is inserted into the uterus by a doctor.
What is definitive contraception?
A definitive form of contraception is sterilisation. With this surgical procedure, fertility is definitively terminated. In principle, you will not be able to have children after sterilisation. A sterilisation is therefore only meant for people who do not want to have a child anymore. Both men and women can be sterilised.
- Male sterilisation: In a vasectomy (male sterilisation), the vas deferens are cut through. As a result, the sperm no longer contains any sperm and no fertilisation can take place. The operation is performed by a urologist.
- Female sterilisation: If a woman opts for sterilisation, the fallopian tubes are sealed off. As a result, fertile eggs can no longer come into contact with the sperm cells. Nowadays a urologist only performs this surgical procedure via two small incisions in the abdominal wall (laparoscopy).
For both men and women, sterilisation is an outpatient procedure, so you can go home the same day.
Alternative method of contraception: periodic abstention
Another way of not getting pregnant, but without the use of regular contraceptives, is periodic abstinence. This calculates when a woman is fertile. During the fertile period there is no sex, or sex with an adhesion barrier (condom or pessary).
There are different ways to calculate the fertile period of a woman:
- Temperature method: Around ovulation, a woman’s body temperature rises a fraction. By measuring the temperature on a daily basis, a personal fertility profile can be calculated. Special thermometers and computers are available to perform the calculations. However, this method can also be done manually. To accurately map the temperature fluctuations, it is necessary to take the temperature at the same time every day. If you deviate from this, the body temperature may already have changed again, making the measurement result less reliable. Factors such as drinking alcohol, not sleeping enough or too much stress can also influence the measurement results.
- Cervical mucus method: Around ovulation, the composition of the endometrium changes. By studying the mucus from the vagina on a daily basis, it is possible to determine whether or not a woman is fertile. It is not always easy to notice the differences in the mucous membrane.
- Calendar method: With a regular cycle, ovulation (and therefore the fertile period) also occurs at regular intervals. By keeping the pattern of the cycle on a calendar, the fertile period can be predicted at some point. An irregular cycle makes it much more difficult to map out the fertile period.
What are the additional risks and side effects?
There may be risks and side effects associated with the use of contraceptives. These differ per contraceptive.
What are the risks and side effects of hormonal contraceptives?
Hormone-based contraceptives can cause side effects. The best known side effects of hormonal contraceptives are:
- slight bleeding outside menstruation (breakthrough bleeding or spotting);
- headache;
- tense or sore breasts;
- weight gain;
- moisture retention;
- mood swings.
Side effects that are rare but serious include cancer and thrombosis. Women who take the pill or use other forms of hormonal contraception have a slightly increased risk of developing these conditions. However, women over the age of 35 who smoke are more likely to experience these side effects. They are not advised to use hormonal contraception. This also applies to women who suffer from certain conditions or who take certain medicines.
What if you miss a dose of hormonal contraceptive?
Hormonal contraceptives are only reliable if they are used very precisely. Have you forgotten to take the pill, for example, or to apply a new ring or patch? Then it is possible that you are no longer optimally protected against pregnancy. This is also the case if you have vomited out the pill or if the patch has accidentally come loose. The body may not have been able to absorb the active ingredients sufficiently. The product information leaflet states exactly what you should do if this situation occurs.
Can you breastfeed when using hormonal contraceptives?
The hormones in contraceptives are also not always suitable for women who are breastfeeding. Contraceptives containing the oestrogen hormone in particular initially reduce the production of breast milk. It is therefore preferable not to use these contraceptives during the first six weeks of breastfeeding. A mini pill with only progesterone has no effect on the production
of breast milk. You can use this pill if you are breastfeeding. The same goes for a coil. However, this contraceptive can only be placed from four to six weeks after childbirth. The hormones you receive can pass into the breast milk and reach the child. This is not necessarily harmful to the child.
What are the risks and side effects of non-hormonal contraceptives?
The use of non-hormonal contraception presents little or no risks or side effects. Placing a copper coil can be painful. Some women with a copper coil also suffer from heavier menstruation. Sterilisation is an operation, involving the usual risks of an operation carries. The reliability of adhesion barriers is significantly reduced if the condom or pessary is not used properly. A condom can tear, for example, or a pessary can move. In addition, the use of certain medications may affect the material of the contraceptive and make it less reliable. Always read the manual carefully before using these contraceptives.
What are the risks and side effects of alternative methods of contraception?
The reliability of the alternative method of contraception (periodic abstinence) is much less than that of regular contraceptives. The calendar and temperature methods require very accurate records. For the cervical smear method, the mucous membrane is not always easy to assess. In addition, sperm cells can survive for several days in the uterus. If the periodic abstinence is initiated too late, there is a chance that an egg will be fertilised.
More women accidentally become pregnant using periodic abstinence as a method of contraception than those using regular contraceptives.